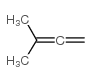
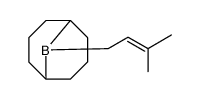
|
~90% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

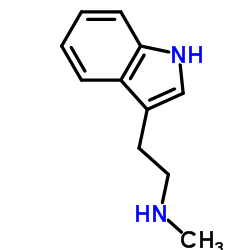
![N-[2-(1H-indol-3-yl)ethyl]-N-methylformamide Structure](https://image.chemsrc.com/caspic/072/54268-27-8.png)
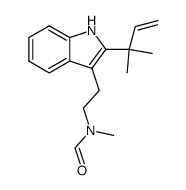
![2-[6-Bromo-2-(2-methyl-3-buten-2-yl)-1H-indol-3-yl]-N-methylethan amine hydrochloride (1:1) Structure](https://image.chemsrc.com/caspic/262/474657-72-2.png)