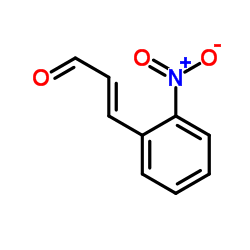
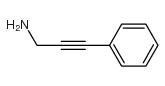
|
~98% |
|
~55% |
|
~73% |
|
~% |
|
~56% |

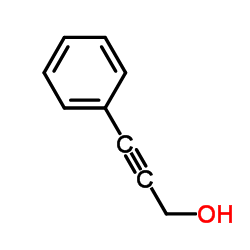
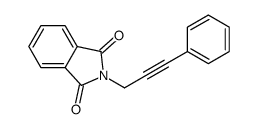

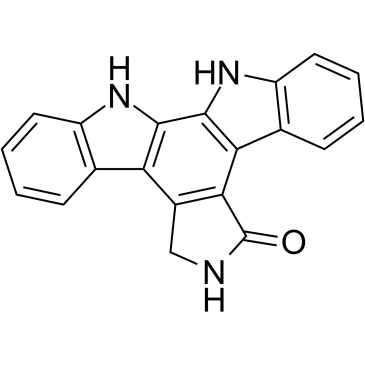
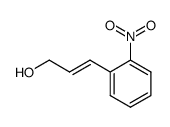
![2-[3-(2-nitrophenyl)prop-2-enyl]isoindole-1,3-dione Structure](https://image.chemsrc.com/caspic/066/75059-03-9.png)