|
~32% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~57%
Detail
|
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~89% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~51% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~97% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

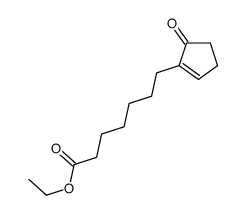
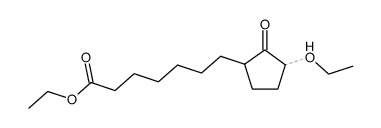



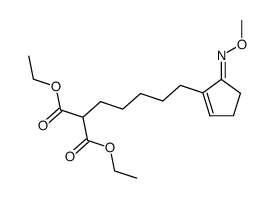

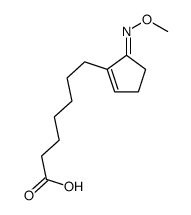
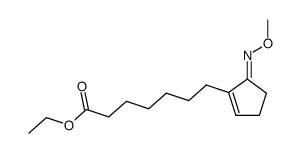
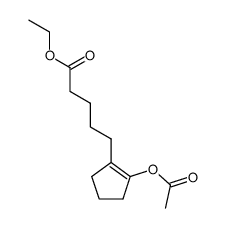
![Methanesulfonic acid 5-{5-[(E)-methoxyimino]-cyclopent-1-enyl}-pentyl ester Structure](https://image.chemsrc.com/caspic/094/52477-93-7.png)
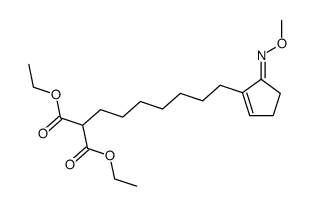
![2-[5-(5-methoxyiminocyclopenten-1-yl)pentyl]propanedioic acid Structure](https://image.chemsrc.com/caspic/018/52477-95-9.png)

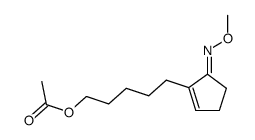
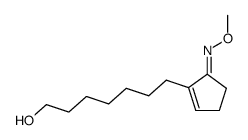
![acetic acid,[2-(5-hydroxypentyl)cyclopenten-1-yl] acetate Structure](https://image.chemsrc.com/caspic/208/52477-90-4.png)
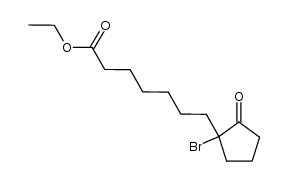

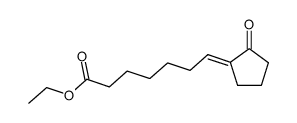
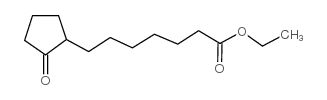
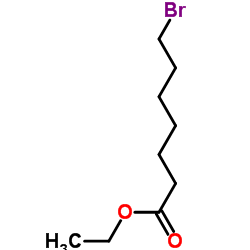
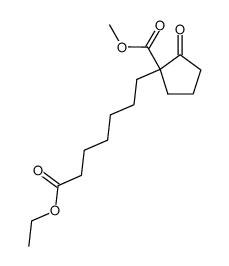

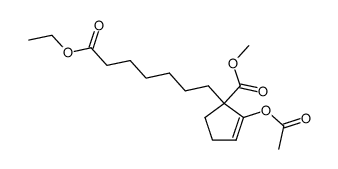

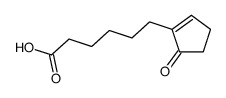
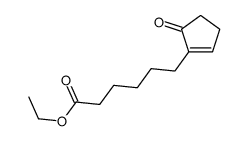
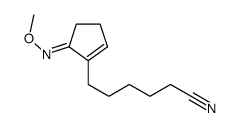
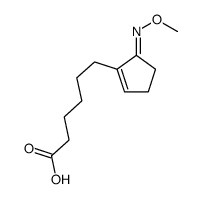
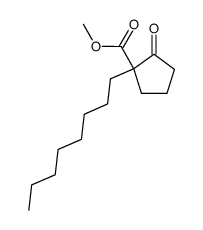

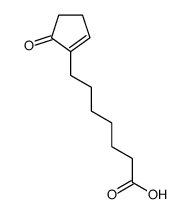
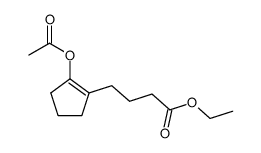
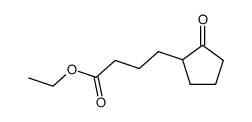
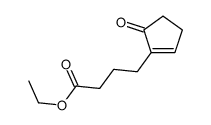
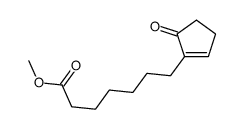
![ethyl 2-[4-(5-oxocyclopenten-1-yl)butoxy]acetate Structure](https://image.chemsrc.com/caspic/283/41302-81-2.png)
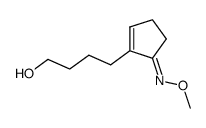
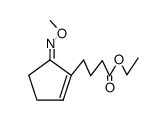
![2-[4-(5-methoxyiminocyclopenten-1-yl)butoxy]acetic acid Structure](https://image.chemsrc.com/caspic/315/60950-68-7.png)
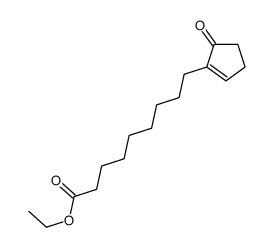
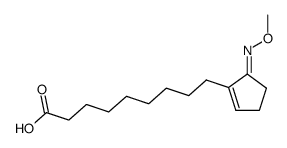
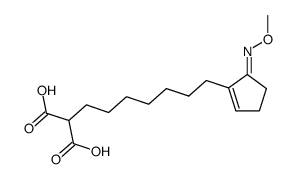

![ethyl 2-[5-(5-oxocyclopenten-1-yl)pentylsulfanyl]acetate Structure](https://image.chemsrc.com/caspic/492/62408-07-5.png)