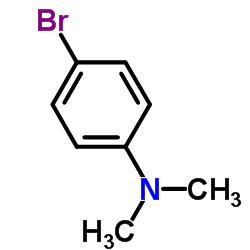
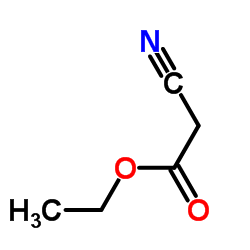
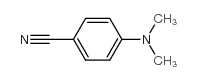
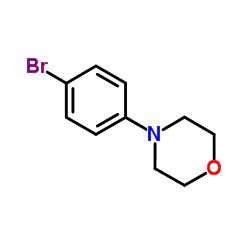
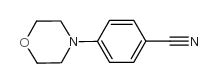
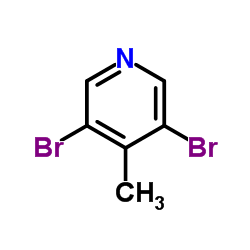
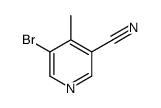
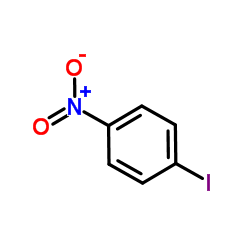
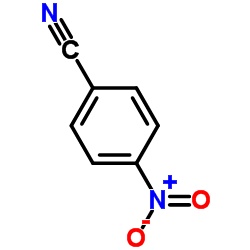
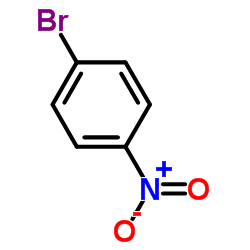
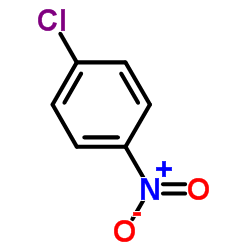
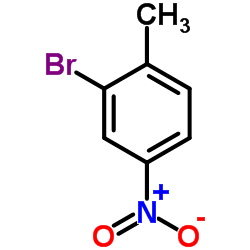
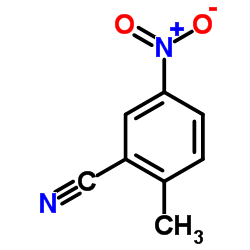
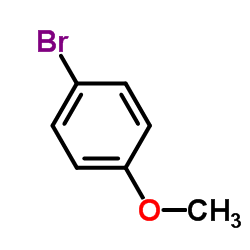
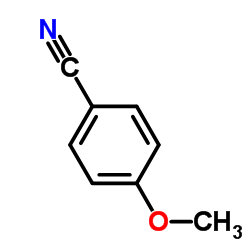
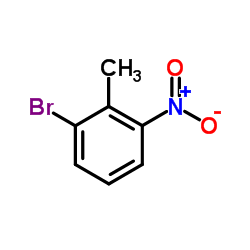
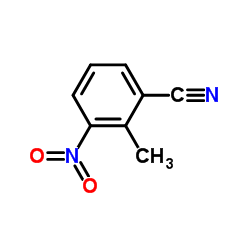
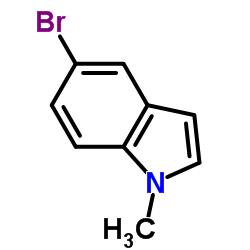
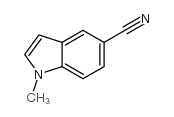
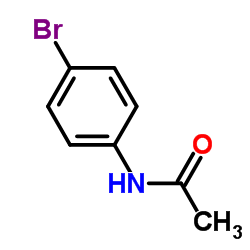
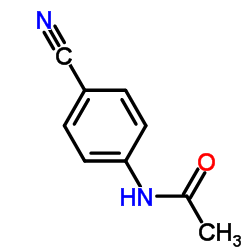
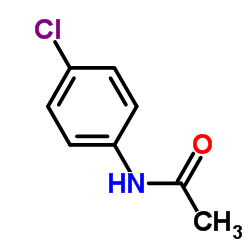
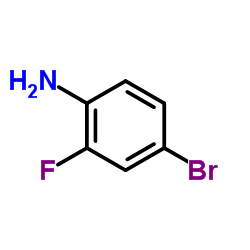
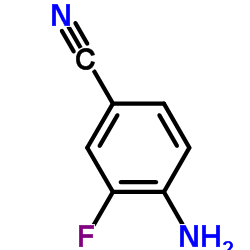
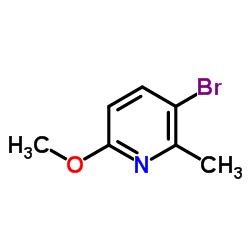
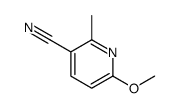
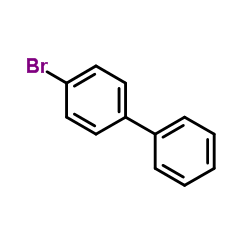
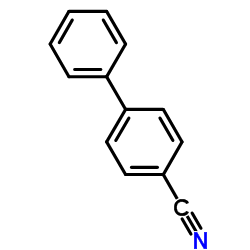
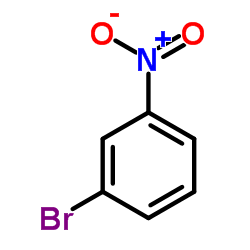
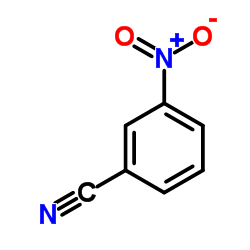
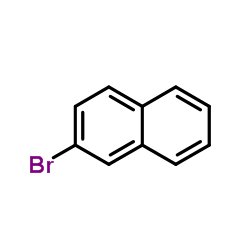
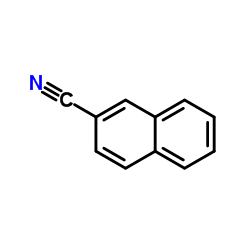
|
~65% |
|
~39% |
|
~83% |
|
~46% |
|
~10% |
|
~89% |
|
~65% |
|
~44% |
|
~96% |
|
~88% |
|
~52% |
|
~38% |
|
~28% |
|
~49% |
|
~77% |
|
~76% |
|
~64% |
|
~93% |
|
~37% |