|
~0% |
|
~0% |
|
~%
Detail
|
![(S)-1-(1-naphthylmethyl)-2-[(E)-2-butenoyl]-5-isopropylpyrazolidin-3-one Structure](https://image.chemsrc.com/caspic/149/1185084-34-7.png)
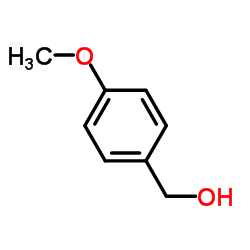


![(S)-1-benzyl-2-[(E)-2-butenoyl]-5-tert-butyl-pyrazolidin-3-one Structure](https://image.chemsrc.com/caspic/204/1185084-28-9.png)
![1-ethyl-2-[(E)-2-butenoyl]-5-isopropyl-pyrazolidin-3-one Structure](https://image.chemsrc.com/caspic/156/1185084-07-4.png)