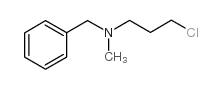
|
~% |
|
~% |
|
~67% |
|
~% |
|
~% |
|
~65% |
|
~% |
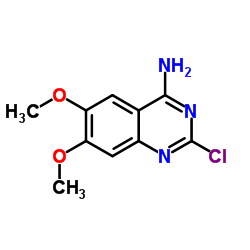
![N-[3-[(4-amino-6,7-dimethoxy-quinazolin-2-yl)-methyl-amino]propyl]benz amide hydrochloride Structure](https://image.chemsrc.com/caspic/077/65189-49-3.png)


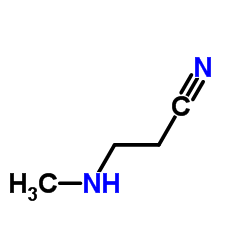
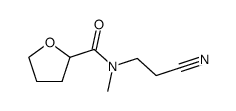
![N-[3-(methylamino)propyl]oxolane-2-carboxamide Structure](https://image.chemsrc.com/caspic/431/81403-67-0.png)