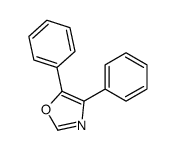
|
~95% |
|
~% |
|
~45% |
|
~% |
|
~65% |
|
~% |
|
~% |
|
~87% |
|
~77% |
|
~% |
|
~90% |
|
~58% |

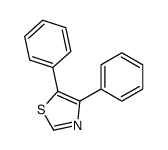
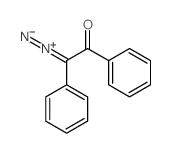
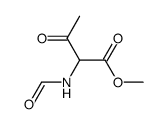
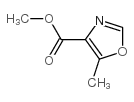
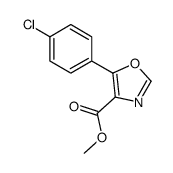
![N-[2-(4-chlorophenyl)-1-methoxycarbonyl-2-oxoethyl]formamide Structure](https://image.chemsrc.com/caspic/422/119826-04-9.png)
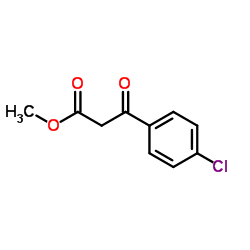
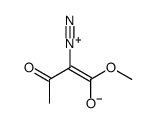
![[2-(4-chlorophenyl)-1-methoxycarbonyl-2-oxo-ethylidene]-imino-azanium Structure](https://image.chemsrc.com/caspic/210/6936-71-6.png)