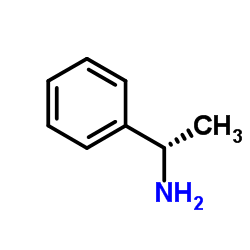
|
~70% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~29% |
|
~% |
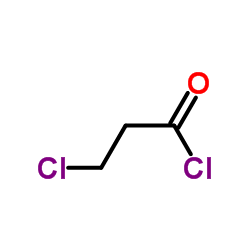
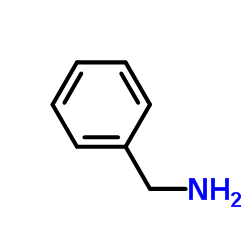
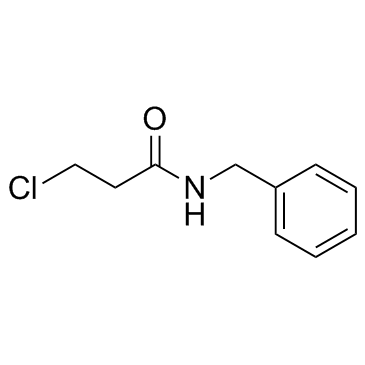
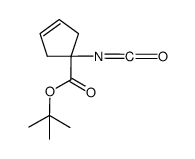
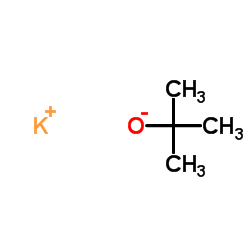
![tert-butyl 1-[(2-methylpropan-2-yl)oxycarbonylamino]cyclopent-3-ene-1-carboxylate Structure](https://image.chemsrc.com/caspic/305/521964-59-0.png)

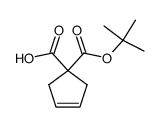


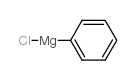

![1S,2S-bis[(1R)-1-phenylethyl]-1,2-diphenyl-1,2-Ethanediamine Structure](https://image.chemsrc.com/caspic/398/156730-49-3.png)