|
~75% |
|
~% |
|
~% |
|
~% |
|
~21% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
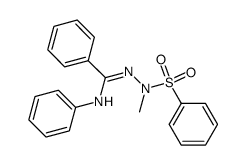
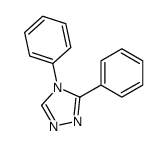
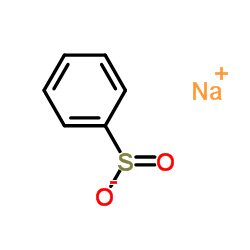
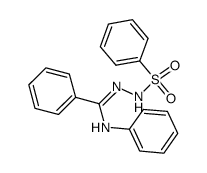

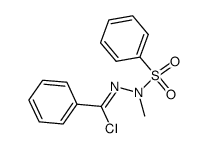
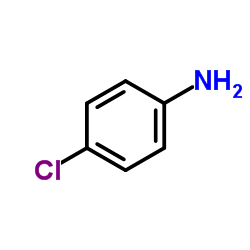
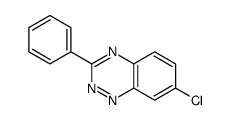


![4-phenyl-3-p-tolyl-4H-[1,2,4]triazole Structure](https://image.chemsrc.com/caspic/222/70187-21-2.png)