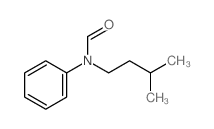
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
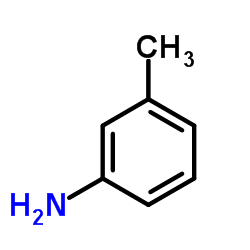




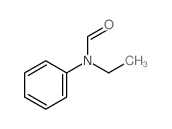

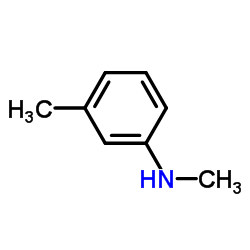


![1-[bis(3-methylbutoxy)methoxy]-3-methyl-butane Structure](https://image.chemsrc.com/caspic/441/5337-70-2.png)