|
~% |
|
~% |
|
~% |
|
~% |
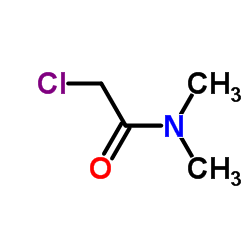

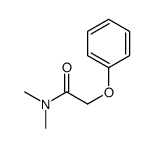
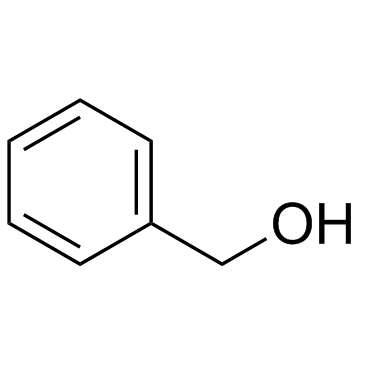
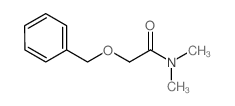
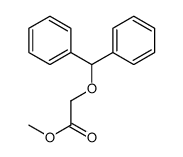
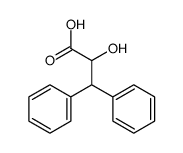
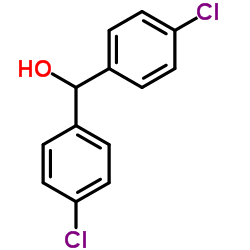
![2-[bis(4-chlorophenyl)methoxy]-N,N-dimethylacetamide Structure](https://image.chemsrc.com/caspic/391/41858-32-6.png)