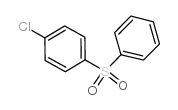
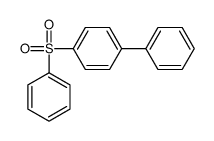
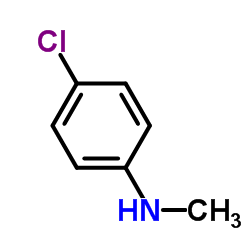
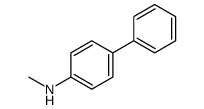
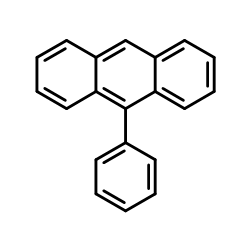
|
~88% |
|
~6% |
|
~81% |
|
~50% |
|
~19% |
|
~97% |
|
~78% |
|
~96% |
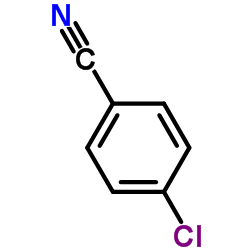
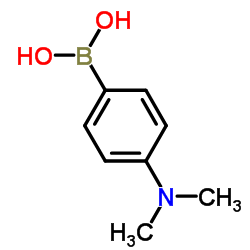
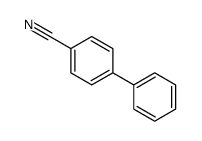
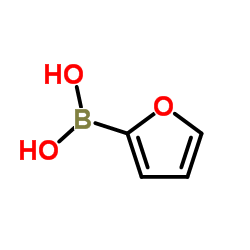

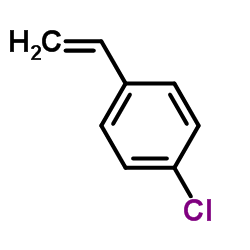
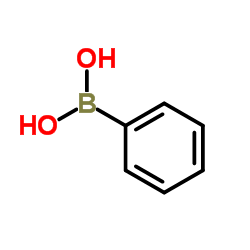
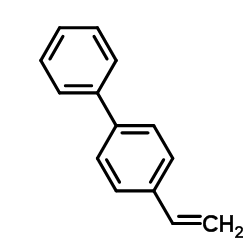
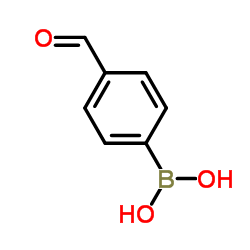
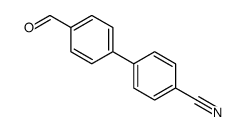
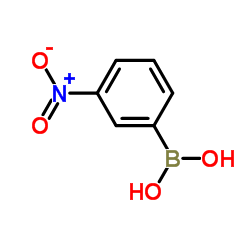
![3'-Nitro-[1,1'-biphenyl]-4-carbonitrile Structure](https://image.chemsrc.com/caspic/203/39117-72-1.png)