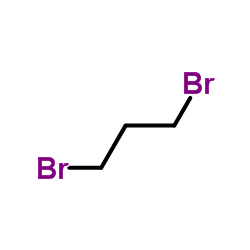
|
~93% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~91% |
![ethyl (αS,γS)-α-{[(1,1-dimethylethoxy)carbonyl]amino}-4-methoxy-3-(3-methoxypropoxy)-γ-(1-methylethyl)benzenepentanoate Structure](https://image.chemsrc.com/caspic/062/172900-76-4.png)
![tert-butyl N-[1-hydroxy-4-[[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl]-5-methylhexan-2-yl]carbamate Structure](https://image.chemsrc.com/caspic/007/172900-82-2.png)
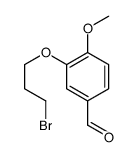

![methyl (2S,4S)-2-amino-4-[4-methoxy-3-(3-methoxypropoxy)benzyl]-5-methylhexanoate Structure](https://image.chemsrc.com/caspic/267/656241-36-0.png)
![(2R,5S)-2,5-dihydro-3,6-dimethoxy-2-{(2S)-2-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-3-methylbutyl}-5-(1-methylethyl)pyrazine Structure](https://image.chemsrc.com/caspic/429/656241-32-6.png)
![(2S)-3,6-diethoxy-2,5-dihydro-2-{(2S)-2-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-3-methylbutyl}-pyrazine Structure](https://image.chemsrc.com/caspic/193/172900-68-4.png)
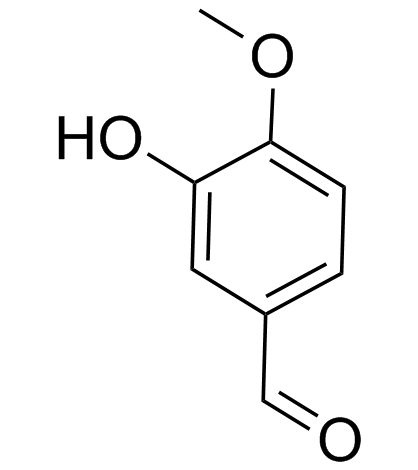
![(4,8-dioxothieno[3,2-f][1]benzothiol-2-yl)methyl acetate Structure](https://image.chemsrc.com/caspic/174/656241-21-3.png)

![(R)-2-[3-(3-METHOXYPROPOXY)-4-METHOXYBENZYL]-3-METHYLBUTAN-1-OL Structure](https://image.chemsrc.com/caspic/300/172900-70-8.png)
![(R)-2-[3-(3-METHOXYPROPOXY)-4-METHOXYBENZYL]-3-METHYL-BUTYRICACID Structure](https://image.chemsrc.com/caspic/262/172900-71-9.png)
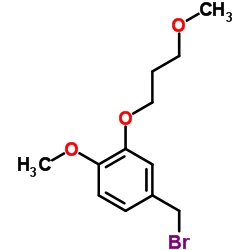
![[4-methoxy-3-(3-methoxypropoxy)phenyl]methanol Structure](https://image.chemsrc.com/caspic/138/172900-74-2.png)
![(4R)-3-{(2R)-2-{[4-methoxy-3-(methoxypropoxy)phenyl]methyl-3-methyl}-1-oxobutyl}-4-(phenylmethyl)oxazolidin-2-one Structure](https://image.chemsrc.com/caspic/214/172900-72-0.png)