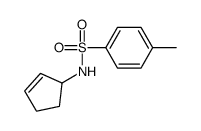
|
~68% |
|
~% |
|
~% |
|
~% |
|
~42% |
|
~% |
|
~% |
![7-Aza-bicyclo[4.1.0]heptane Structure](https://image.chemsrc.com/caspic/031/286-18-0.png)

![tert-butyl 7-azabicyclo[4.1.0]heptane-7-carboxylate Structure](https://image.chemsrc.com/caspic/317/153789-13-0.png)

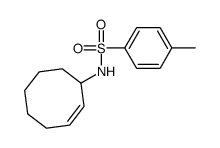

![6-Tosyl-6-azabicyclo[3.1.0]hexane Structure](https://image.chemsrc.com/caspic/208/81097-48-5.png)