|
~84% |
|
~74% |
|
~66% |
|
~36% |
|
~75% |
|
~57% |
|
~88% |
![2-[6-methoxycarbonyl-2-(4-methylphenyl)imidazo[1,2-a]pyridin-3-yl]acetic acid Structure](https://image.chemsrc.com/caspic/363/917252-80-3.png)

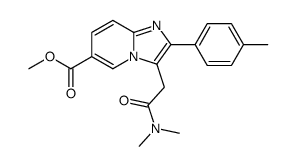


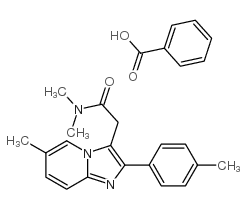
![2-[4-(Ethoxycarbonyl)phenyl]-6-methyl-imidazo[1,2-a]pyridine-3-acetic Acid Structure](https://image.chemsrc.com/caspic/361/1025962-20-2.png)

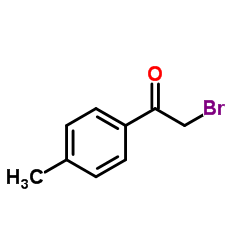
![Methyl 2-(4-methylphenyl)imidazo[1,2-a]pyridine-6-carboxylate Structure](https://image.chemsrc.com/caspic/266/917252-78-9.png)
![methyl 3-tert-butoxycarbonylmethyl-2-(4-methylphenyl)imidazo[1,2-a]pyridine-6-carboxylate Structure](https://image.chemsrc.com/caspic/218/917252-79-0.png)
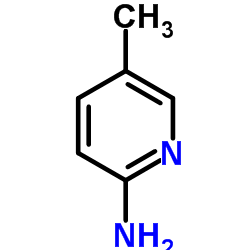
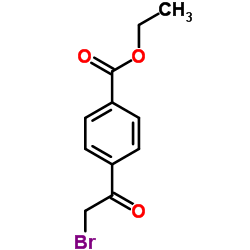
![2-[4-(Ethoxycarbonyl)phenyl]-6-methyl-imidazo[1,2-a]pyridine Structure](https://image.chemsrc.com/caspic/394/109461-69-0.png)