|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~79% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~80% |
|
~% |
|
~85% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~80% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
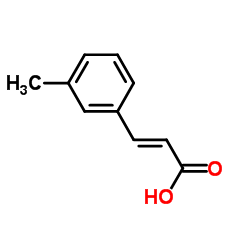
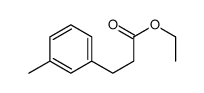
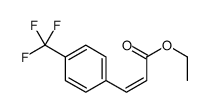
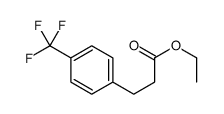
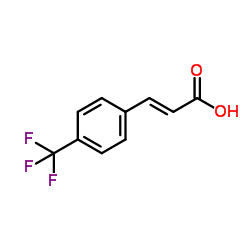

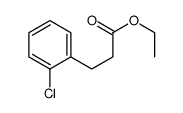
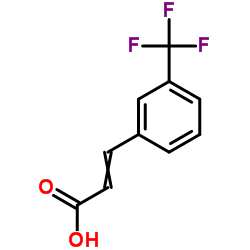
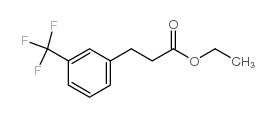
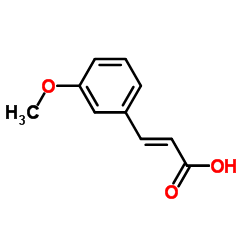
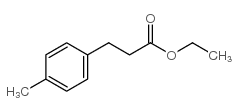
![2,4(1H,3H)-Pyrimidinedione,5-[(3-methoxyphenyl)methyl] Structure](https://image.chemsrc.com/caspic/280/28495-76-3.png)
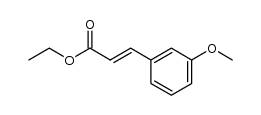
![4(1H)-Pyrimidinone,2,3-dihydro-5-[(3-methoxyphenyl)methyl]-2-thioxo Structure](https://image.chemsrc.com/caspic/094/28495-90-1.png)
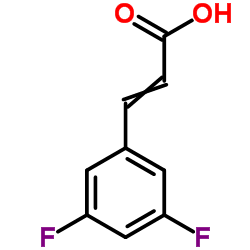
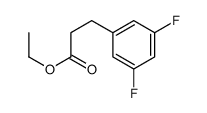
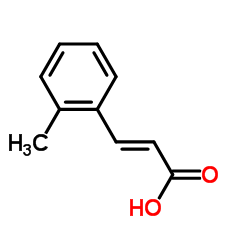
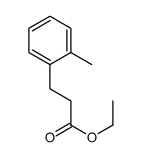

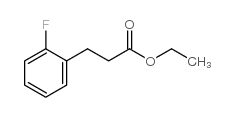
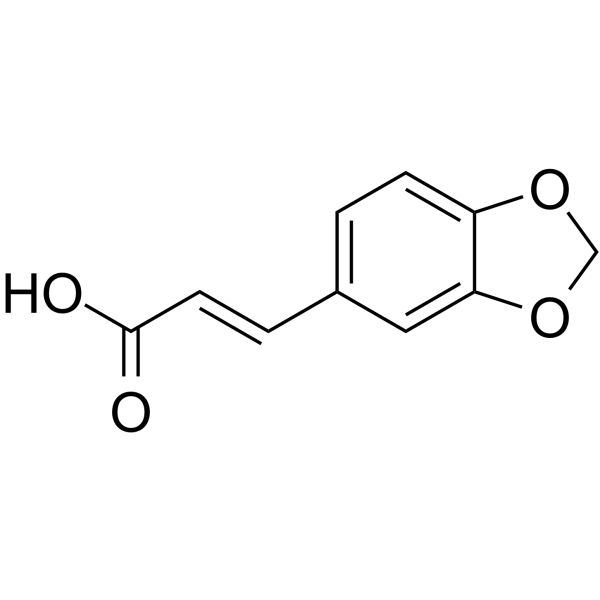
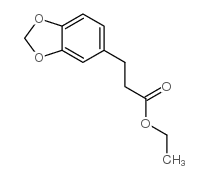

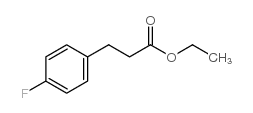
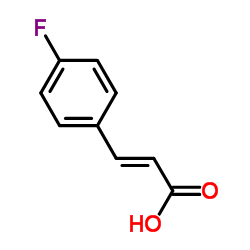
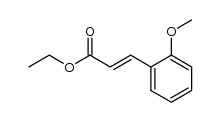
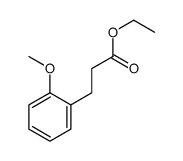
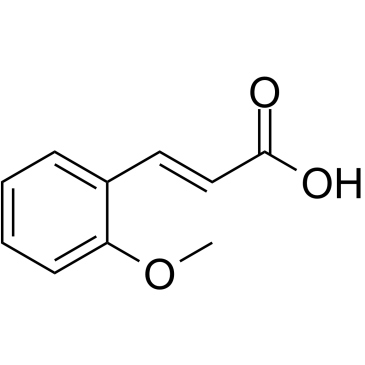

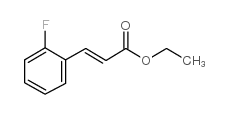

![5-[(4-chlorophenyl)methyl]-2-sulfanylidene-1H-pyrimidin-4-one Structure](https://image.chemsrc.com/caspic/131/63204-27-3.png)
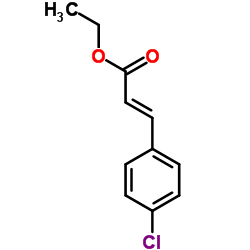

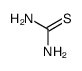
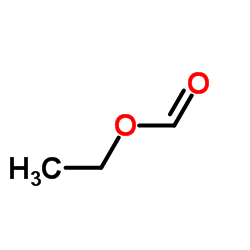
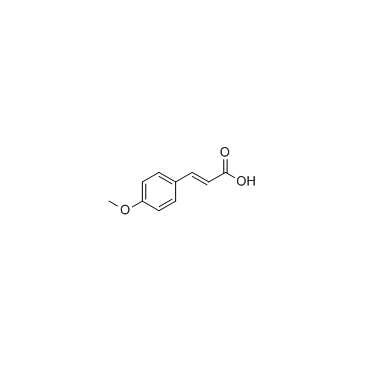
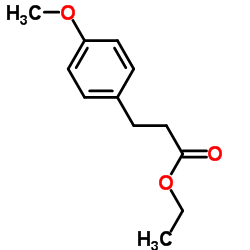
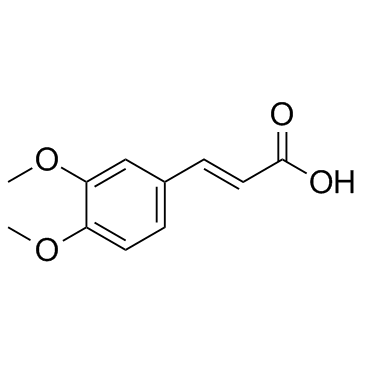
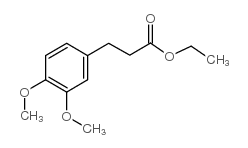
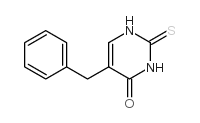
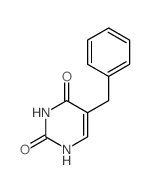
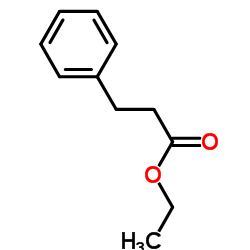
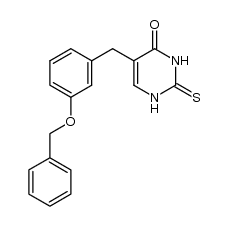
![2,4(1H,3H)-Pyrimidinedione,5-[[3-(phenylmethoxy)phenyl]methyl] Structure](https://image.chemsrc.com/caspic/311/28495-80-9.png)
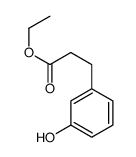

![ethyl 3-[3-(benzyloxy)phenyl]propanoate Structure](https://image.chemsrc.com/caspic/080/75849-10-4.png)
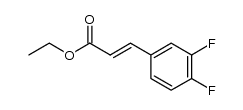
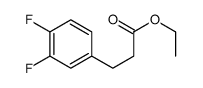
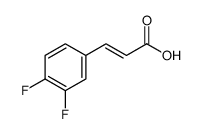

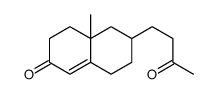
![4(1H)-Pyrimidinone,5-[(3-ethoxyphenyl)methyl]-2,3-dihydro-2-thioxo Structure](https://image.chemsrc.com/caspic/056/28495-91-2.png)

![2,4(1H,3H)-Pyrimidinedione,5-[(3-ethoxyphenyl)methyl] Structure](https://image.chemsrc.com/caspic/204/28495-78-5.png)
![5-[(4-methylphenyl)methyl]-2-sulfanylidene-1H-pyrimidin-4-one Structure](https://image.chemsrc.com/caspic/200/63204-30-8.png)