|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
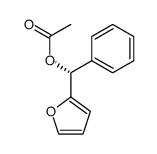
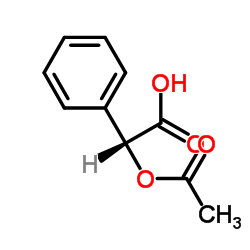



![(2R)-2-[tert-butyl(dimethyl)silyl]oxy-3-methylbutanoic acid Structure](https://image.chemsrc.com/caspic/363/119619-47-5.png)
