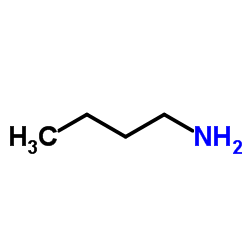
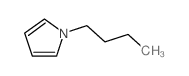
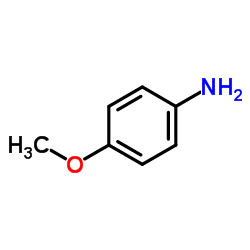
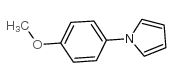
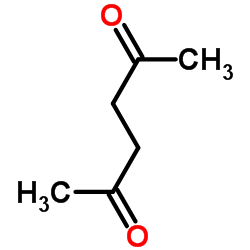
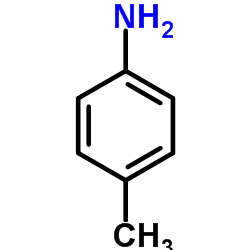
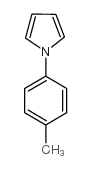
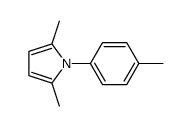
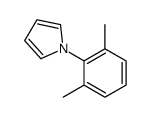
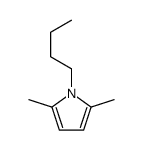
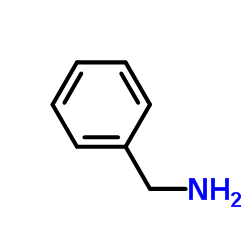
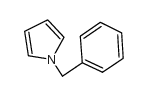
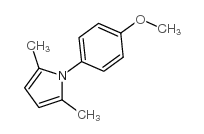
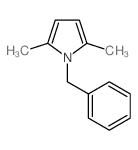
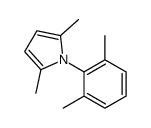
|
~2% |
|
~4% |
|
~3% |
|
~4% |
|
~99% |
|
~3% |
|
~2% |
|
~2% |
|
~98% |
|
~% |
|
~65% |