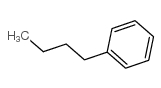
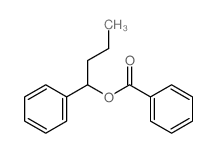
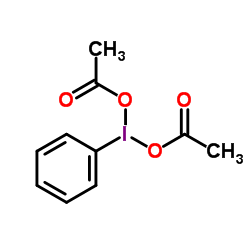
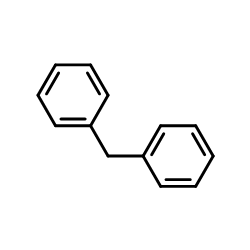
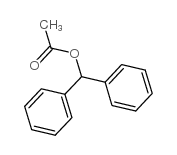
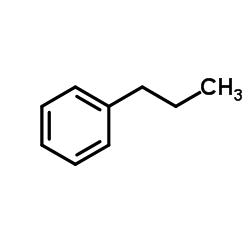
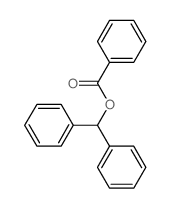
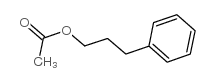
|
~57% |
|
~61% |
|
~40% |
|
~49% |
|
~30% |
|
~64% |