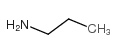
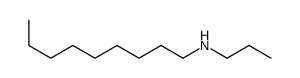
|
~94% |
|
~21% |
|
~16%
Detail
|


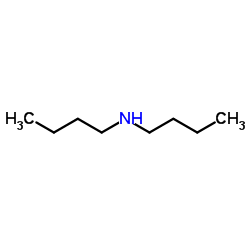
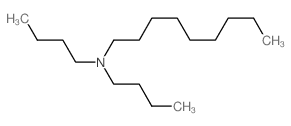
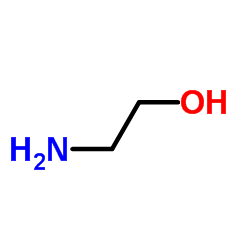
![2-[di(nonyl)amino]ethanol Structure](https://image.chemsrc.com/caspic/065/17618-19-8.png)