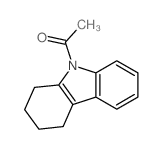
|
~84% |
|
~% |
|
~% |
|
~85% |
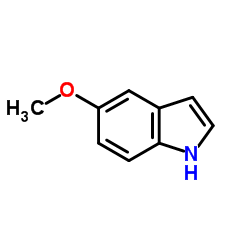

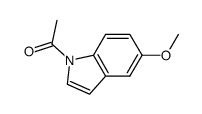
![methyl N-[2-(1H-indol-3-yl)ethyl]carbamate Structure](https://image.chemsrc.com/caspic/015/58635-45-3.png)
![methyl N-[2-(1-acetylindol-3-yl)ethyl]carbamate Structure](https://image.chemsrc.com/caspic/163/88368-97-2.png)