|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
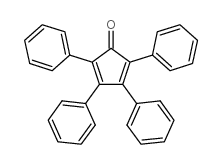
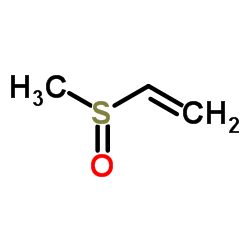

![(5R,6R)-1,6,7,8-tetraphenyl-3-thiatricyclo[4.2.1.02,5]non-7-en-9-one 3,3-dioxide Structure](https://image.chemsrc.com/caspic/392/32513-58-9.png)
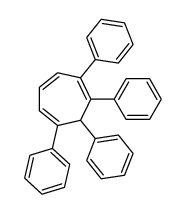
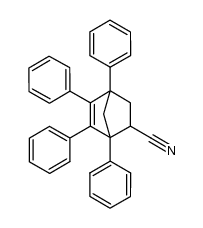
![(1,2,3,4-tetraphenyl-6-bicyclo[2.2.1]hept-2-enyl)methanamine Structure](https://image.chemsrc.com/caspic/261/32513-66-9.png)
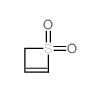
![2H-Cyclopenta[l]phenanthren-2-one,1,3-diphenyl Structure](https://image.chemsrc.com/caspic/384/5660-91-3.png)
![9,13-diphenyl-11h-cyclohepta[l]phenanthrene Structure](https://image.chemsrc.com/caspic/496/32513-33-0.png)
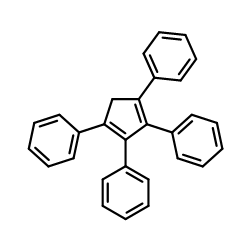
![1,5,6,7-tetraphenylbicyclo[3.2.1]octa-3,6-diene Structure](https://image.chemsrc.com/caspic/344/32513-59-0.png)