|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~89% |
|
~% |
|
~% |

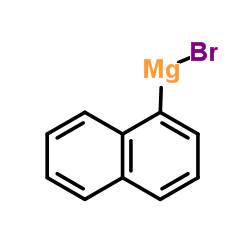
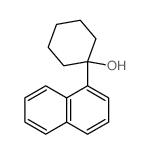

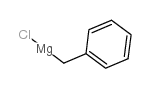
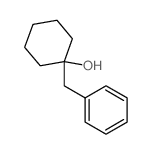
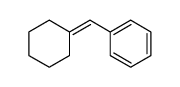
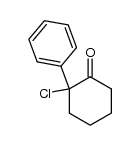
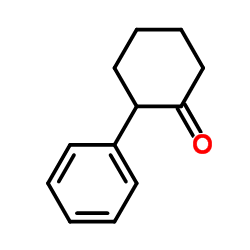
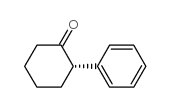


![7-Oxabicyclo[4.1.0]heptane, 1-(phenylmethyl) Structure](https://image.chemsrc.com/caspic/376/7583-78-0.png)
![6-(4-methoxyphenyl)-7-oxabicyclo[4.1.0]heptane Structure](https://image.chemsrc.com/caspic/021/43050-16-4.png)
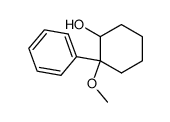

![1-phenyl-7-oxa-bicyclo[4.1.0]heptane Structure](https://image.chemsrc.com/caspic/246/5775-23-5.png)