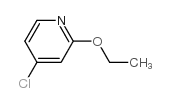
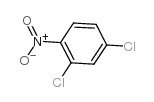
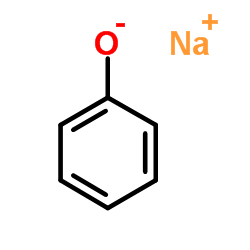
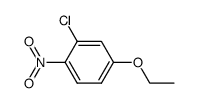
|
~30% |
|
~90% |
|
~61% |
|
~11%
Detail
|