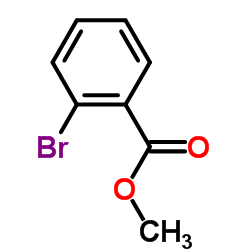
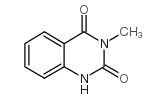
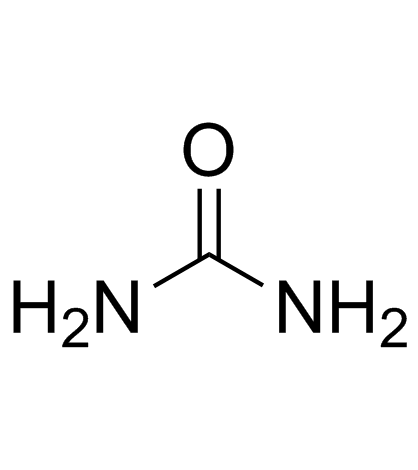
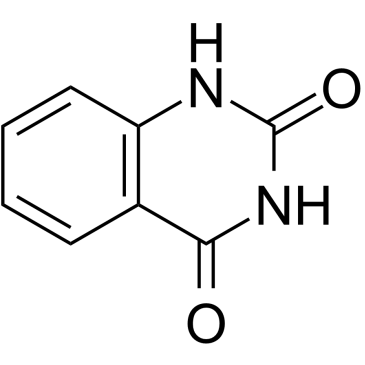
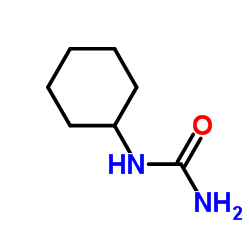
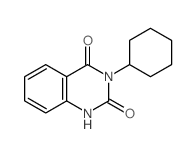
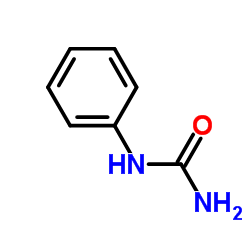
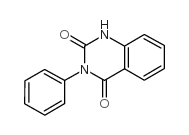
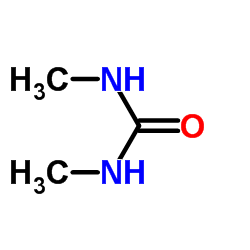
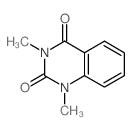
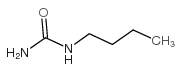
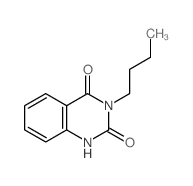
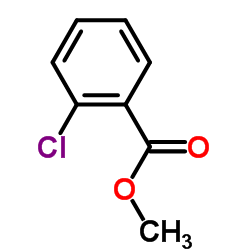
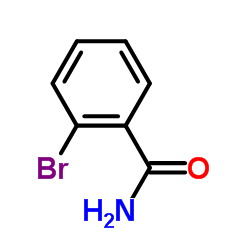
|
~97% |
|
~86% |
|
~99% |
|
~61% |
|
~88% |
|
~94% |
|
~33% |
|
~21% |