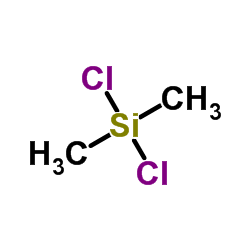
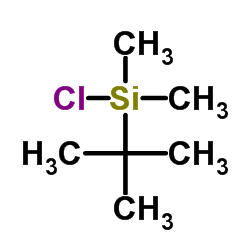
|
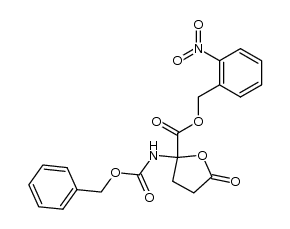
~38% |
|
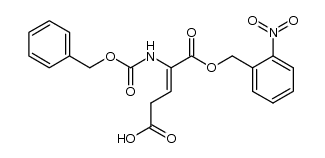
~86% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
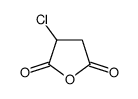
~89% |
|
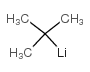
~59% |
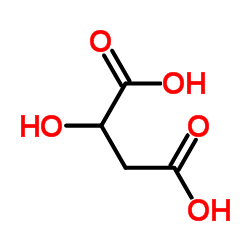
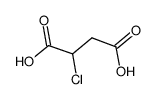
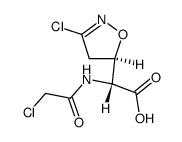
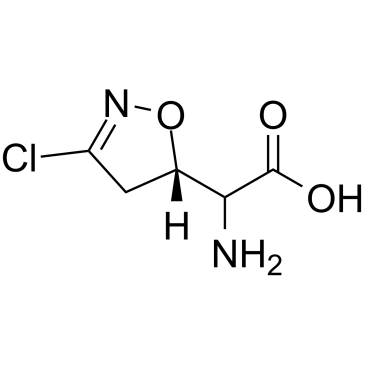
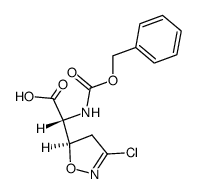

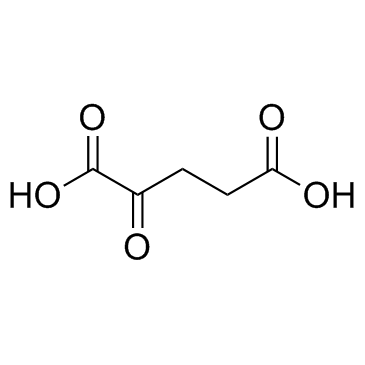
![2-Furancarboxylicacid,tetrahydro-5-oxo-2-[[(phenylmethoxy)carbonyl]amino]-(9CI) Structure](https://image.chemsrc.com/caspic/119/104754-51-0.png)