|
~89% |
|
~68% |
|
~% |
|
~% |
|
~% |
|
~96% |
|
~% |




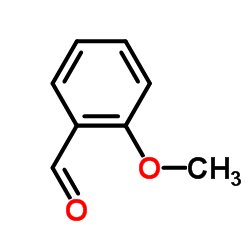
![(-)-(βR)-β-{[(1E)-(2-methoxyphenyl)methylene]amino}benzeneethanol Structure](https://image.chemsrc.com/caspic/198/702684-12-6.png)
![1-[3,5-DI(TERT-BUTYL)-2-HYDROXYPHENYL]ETHAN-1-ONE Structure](https://image.chemsrc.com/caspic/094/37456-29-4.png)
