|
~% |
|
~53% |
|
~% |
|
~81% |
|
~% |
|
~% |
|
~91% |
|
~% |
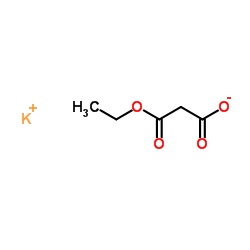
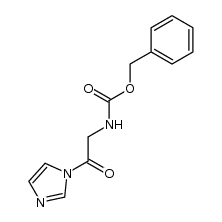
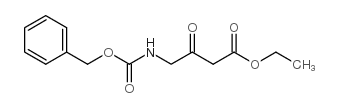
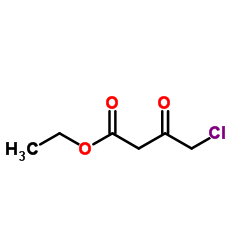
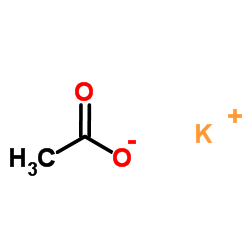
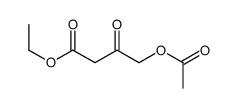
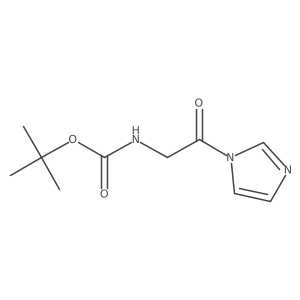
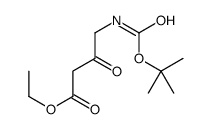
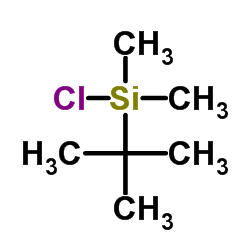
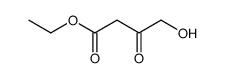
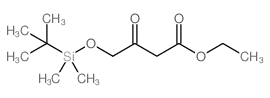
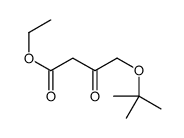
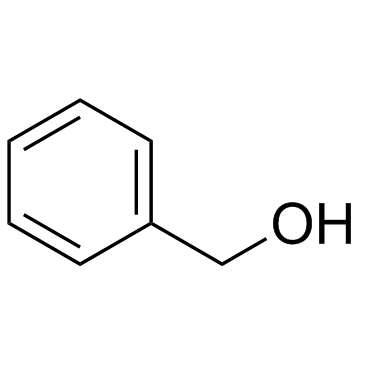
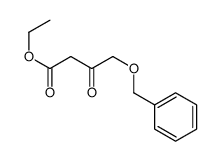
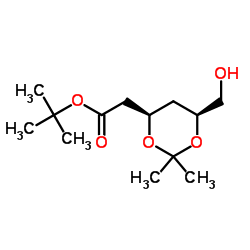

![(4R-Cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-aceticacid,1,1-dimethylethylester Structure](https://image.chemsrc.com/caspic/206/154026-95-6.png)