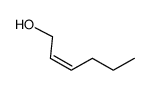
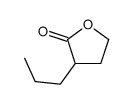
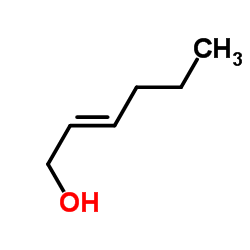
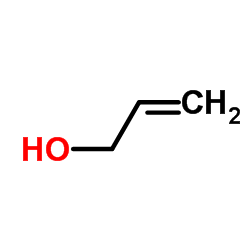
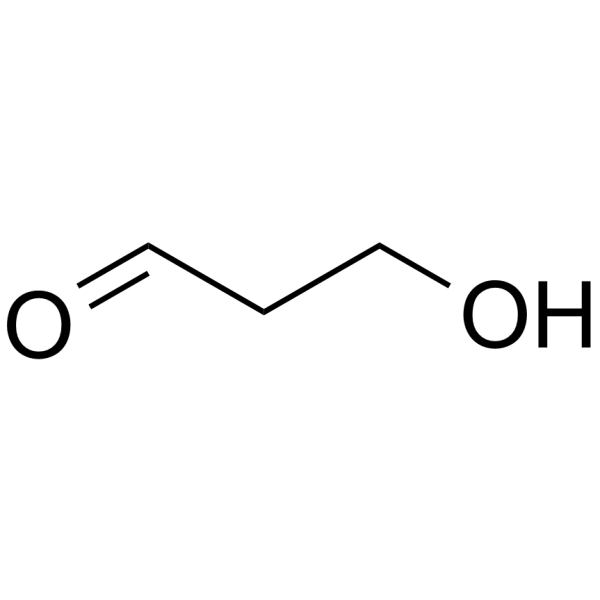
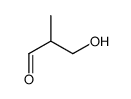
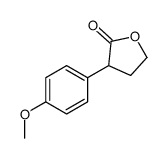
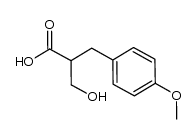
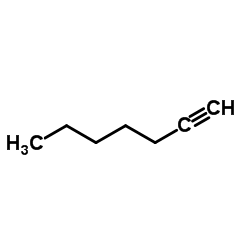
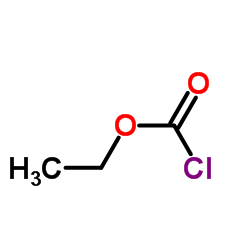
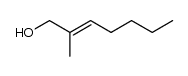
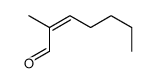
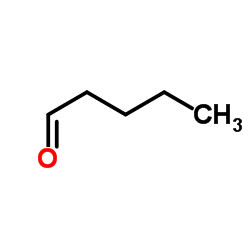
|
~24% |
|
~0% |
|
~0% |
|
~% |
|
~0% |
|
~40% |
|
~57% |
|
~% |
|
~% |
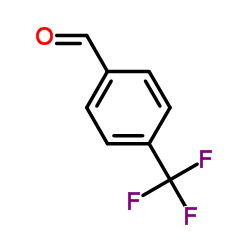
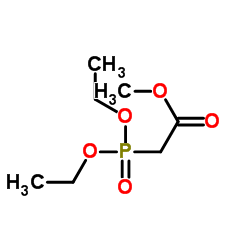
![Methyl (2E)-3-[4-(trifluoromethyl)phenyl]acrylate Structure](https://image.chemsrc.com/caspic/135/20754-22-7.png)