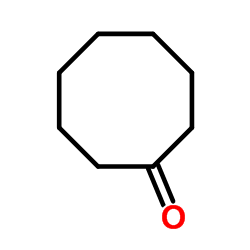
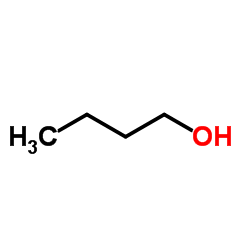
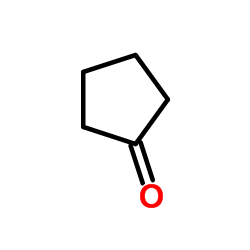
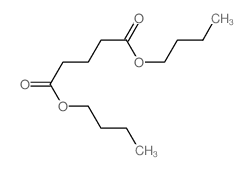
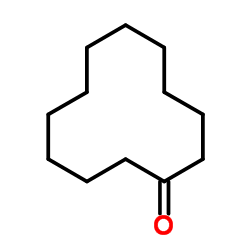
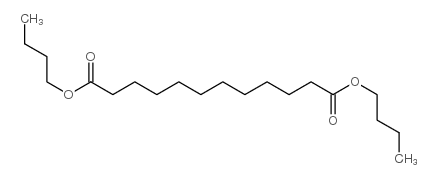
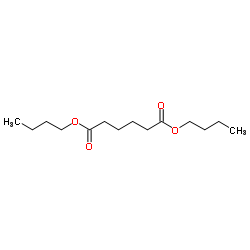
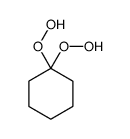
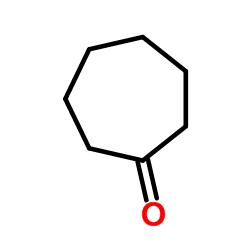
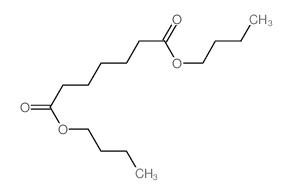
|
~66% |
|
~55% |
|
~70% |
|
~66% |
|
~69% |
|
~68% |