|
~74% |
|
~90% |
|
~94% |
|
~94% |
|
~% |
|
~94% |
|
~86% |
|
~92% |
|
~83% |
|
~80% |
|
~% |
|
~84% |
|
~89% |
|
~86% |
|
~58% |
|
~79% |
|
~94% |
|
~77% |
|
~88% |
|
~% |
|
~81% |
|
~77% |
|
~% |
|
~85% |
|
~77% |
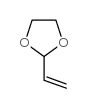
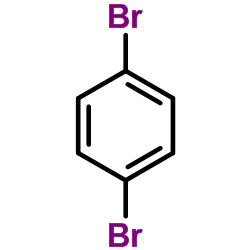
![N-[4-[(2-naphthalen-2-yloxyacetyl)amino]phenyl]butanamide Structure](https://image.chemsrc.com/caspic/184/6082-86-6.png)
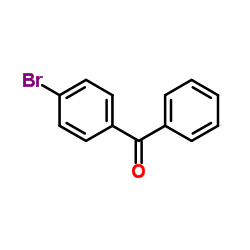
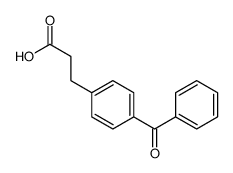
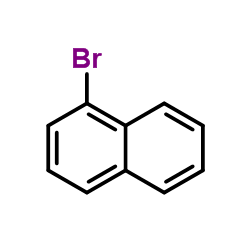
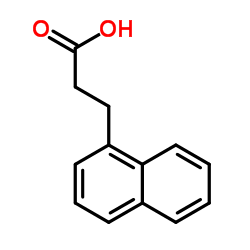
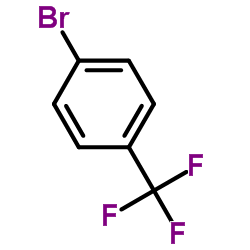
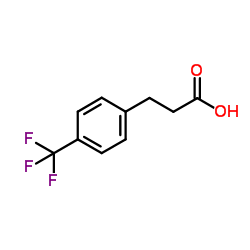
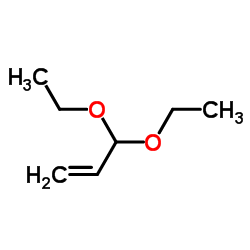
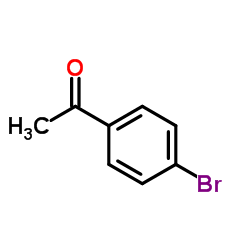
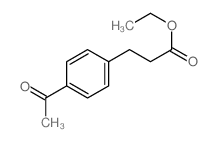

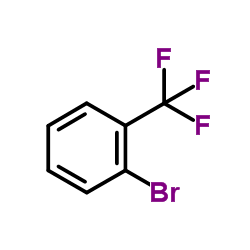
![3-[2-(Trifluoromethyl)phenyl]propanoic acid Structure](https://image.chemsrc.com/caspic/303/94022-99-8.png)

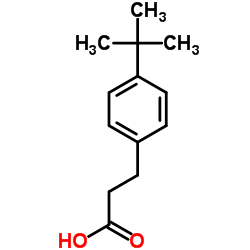
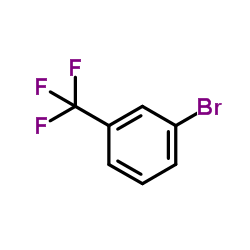
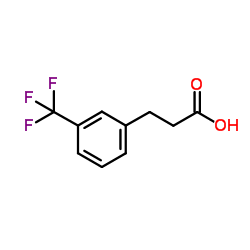
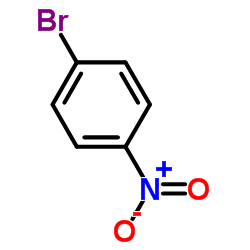
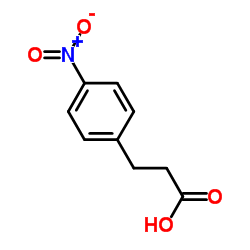
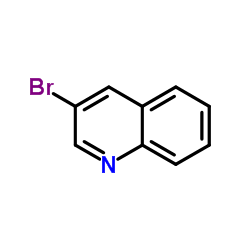

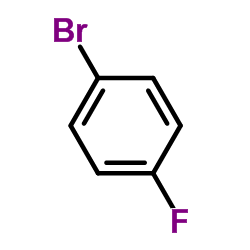
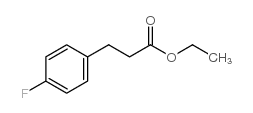
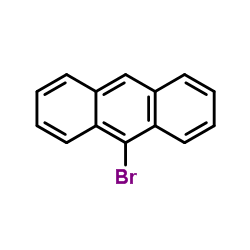
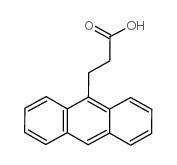
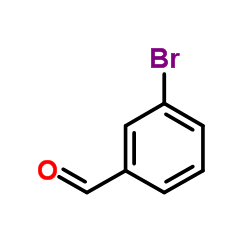
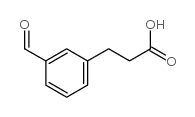
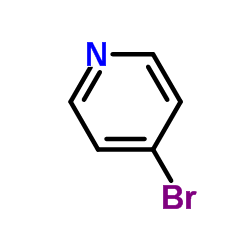
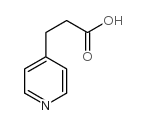
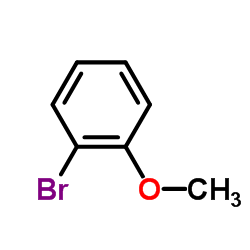
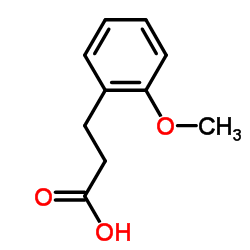
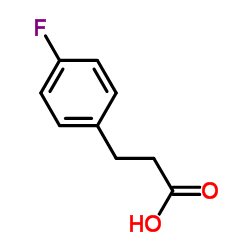
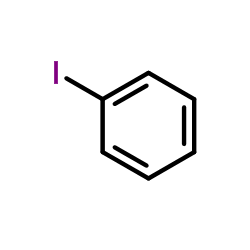
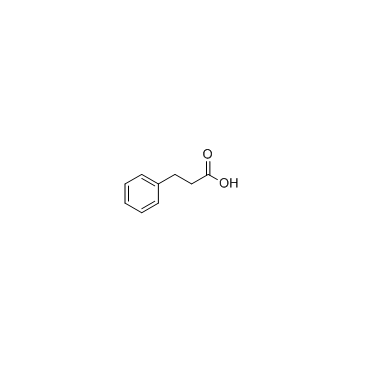
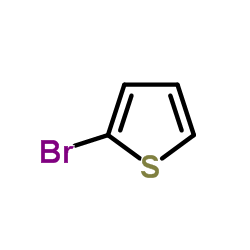
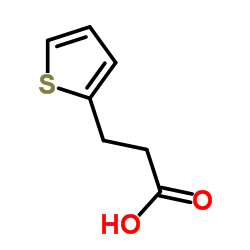
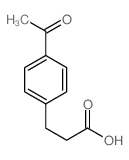
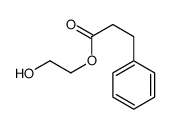
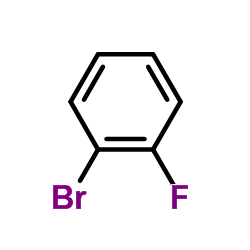

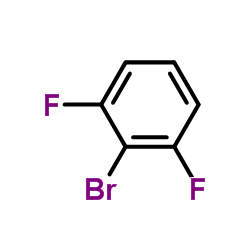
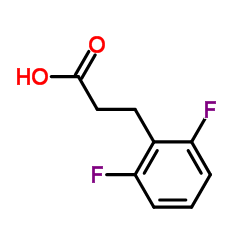
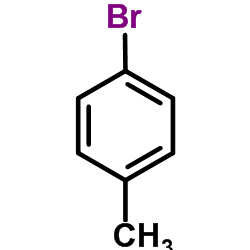
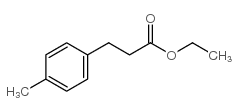

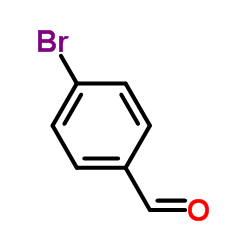
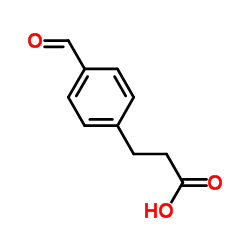
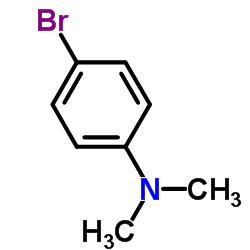
![3-[4-(dimethylamino)phenyl]propanoic acid Structure](https://image.chemsrc.com/caspic/340/73718-09-9.png)