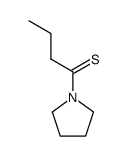
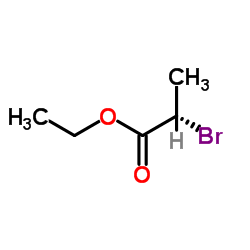
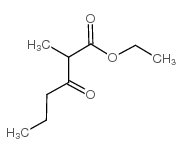
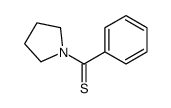
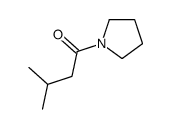
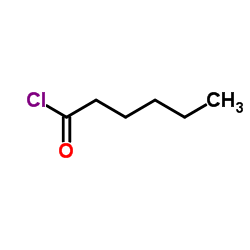
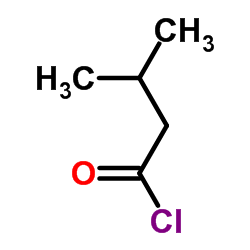
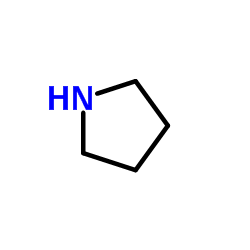
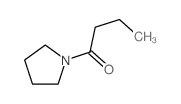
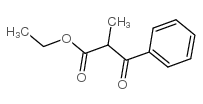
|
~60% |
|
~30% |
|
~40% |
|
~61% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~68% |