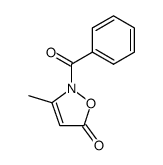
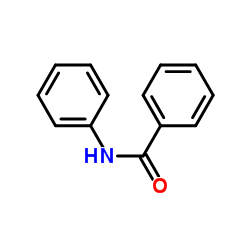
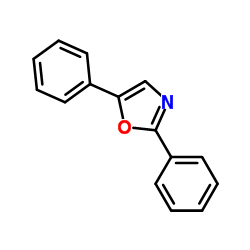
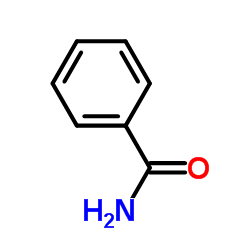
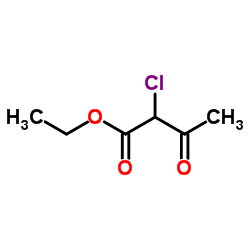
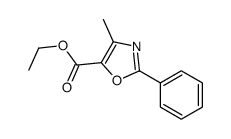
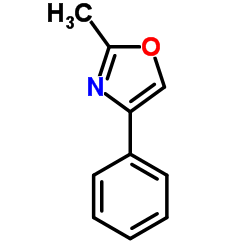
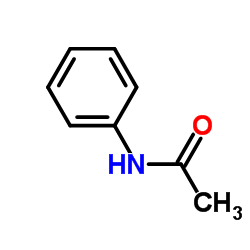
|
~95% |
|
~58% |
|
~19% |
|
~97% |