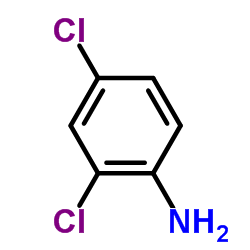
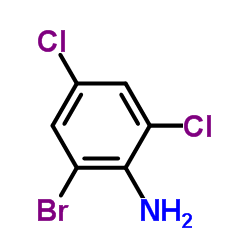
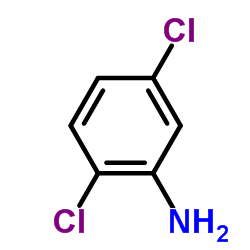
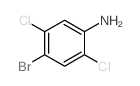
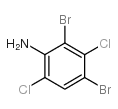
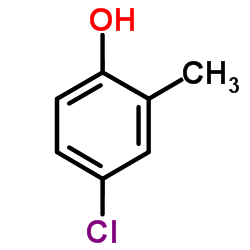
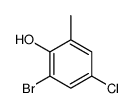
|
~89% |
|
~9% |
|
~19% |
|
~59% |