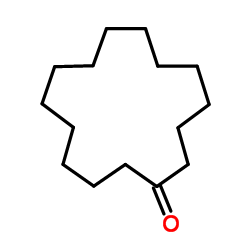
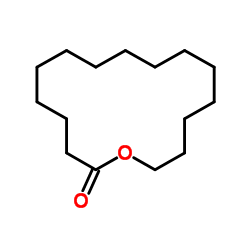
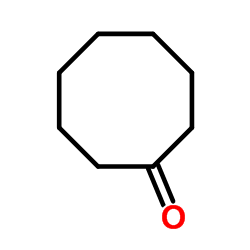
|
~% |
|
~% |
|
~% |
|
~% |
|
~70% |
|
~42% |
|
~% |
|
~% |
![Bis[cyclohexylidene]peroxide Structure](https://image.chemsrc.com/caspic/442/183-84-6.png)

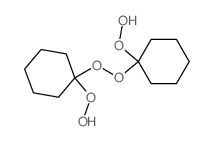
![Cyclohexanol,1-[(1-hydroperoxycyclohexyl)dioxy] Structure](https://image.chemsrc.com/caspic/324/78-18-2.png)