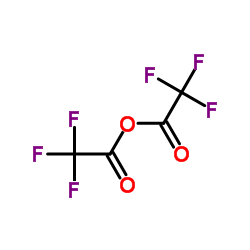
|
~% |
|
~% |
|
~% |
|
~97% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~96% |
|
~% |
|
~% |
|
~% |
|
~95% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~98% |
|
~72% |
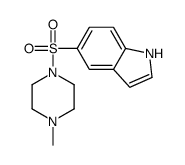
![5-[(4-Methylpiperazin-1-yl)sulfonyl]indoline Structure](https://image.chemsrc.com/caspic/135/519148-71-1.png)



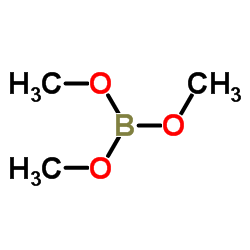
![1H-Indole-1-carboxylic acid, 2-borono-5-[(4-methyl-1-piperazinyl)sulfonyl]-, 1-(1,1-dimethylethyl) ester Structure](https://image.chemsrc.com/caspic/021/519148-74-4.png)