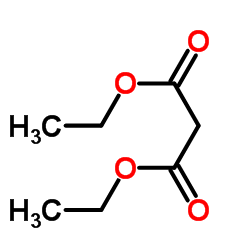
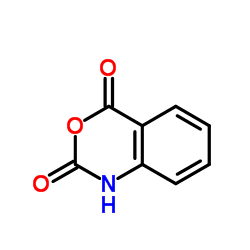
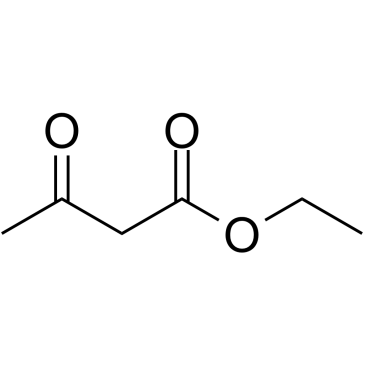
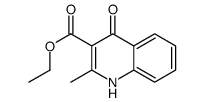
|
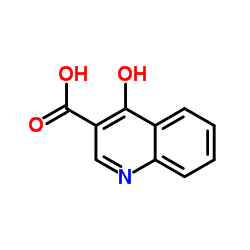
~80% |
|
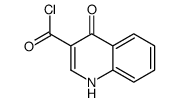
~71% |
|
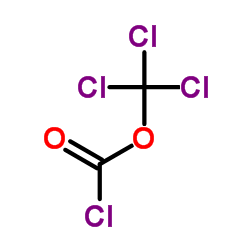
~% |
|
~10% |
![1-PHENYL-1H-PYRIDO[2,3-D][1,3]OXAZINE-2,4-DIONE Structure](https://image.chemsrc.com/caspic/374/138305-19-8.png)