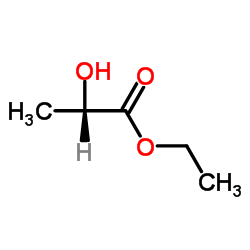
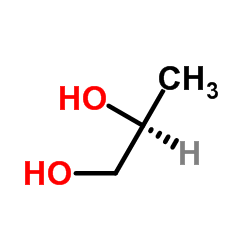
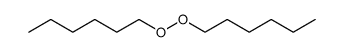
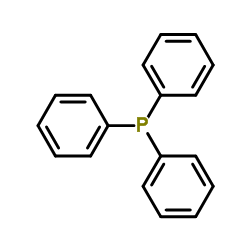
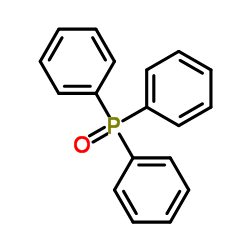
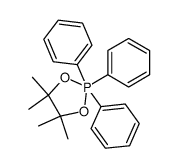
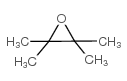
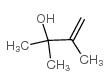
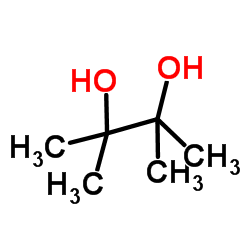
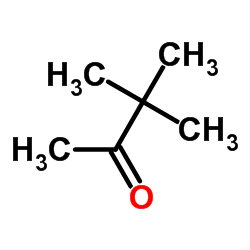
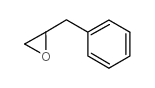
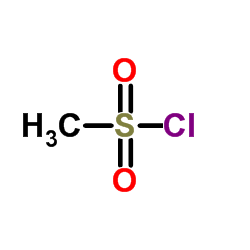
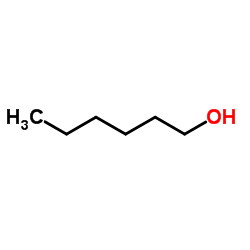
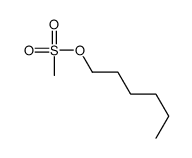
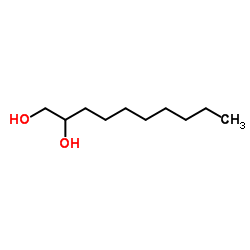
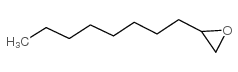
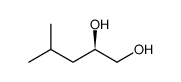
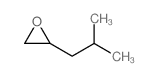
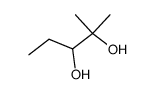
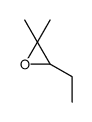
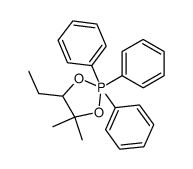
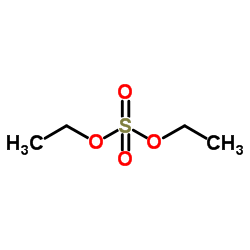
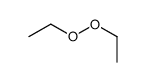
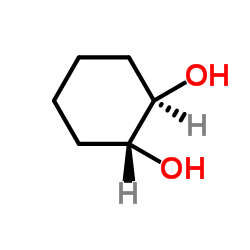
|
~% |
|
~0% |
|
~9% |
|
~92% |
|
~% |
|
~10% |
|
~99% |
|
~58% |
|
~% |
|
~96% |
|
~81% |
|
~% |
|
~60% |
|
~61% |
|
~99% |