|
~96% |
|
~87% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~99% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~73% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~96% |



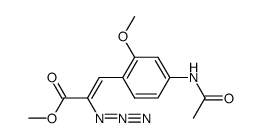



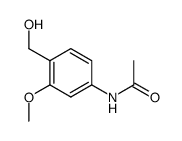
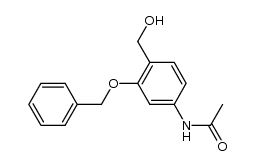
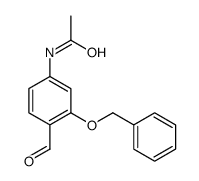
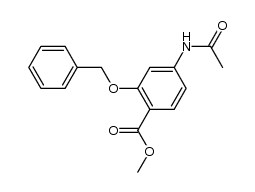
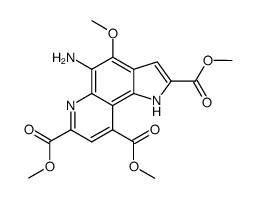
![4,5-Dioxo-4,5-dihydro-1H-pyrrol[2,3-f]quinoline-2,7,9-tricarboxylic acid trimethyl ester Structure](https://image.chemsrc.com/caspic/422/74447-88-4.png)

