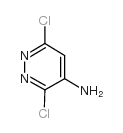
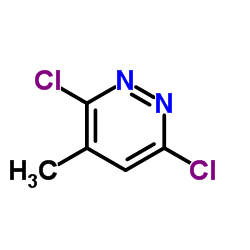
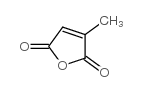
|
~86% |
|
~61% |
|
~81% |
|
~% |
|
~% |
|
~68% |
|
~96% |
|
~76% |
|
~77% |
|
~% |
|
~70% |
|
~% |
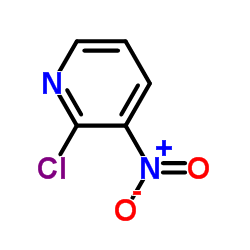
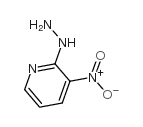


![1,2,4-Triazolo[4,3-b]pyridazin-8-amine,6-chloro Structure](https://image.chemsrc.com/caspic/274/6698-57-3.png)
![1,2,4-Triazolo[4,3-a]pyridine,8-nitro Structure](https://image.chemsrc.com/caspic/065/31040-09-2.png)
![[1,2,4]Triazolo[1,5-a]pyridine,8-nitro Structure](https://image.chemsrc.com/caspic/396/31040-18-3.png)
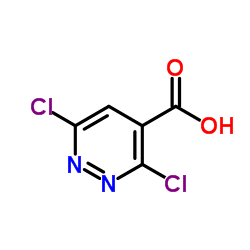
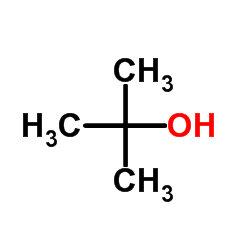
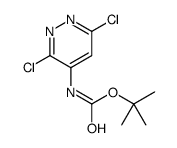
![1,2,7,8-tetrazabicyclo[4.3.0]nona-2,4,6,8-tetraen-5-amine Structure](https://image.chemsrc.com/caspic/347/6583-39-7.png)