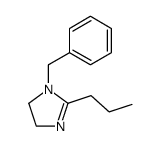
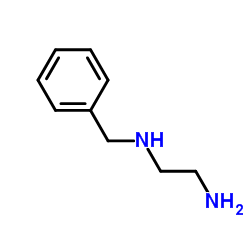
|
~98% |
|
~% |
|
~% |
|
~54% |
|
~% |
|
~97% |
|
~97% |
|
~94% |
|
~% |

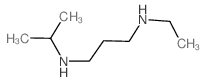
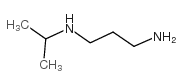

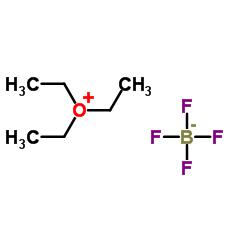
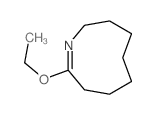

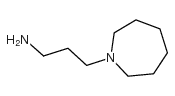

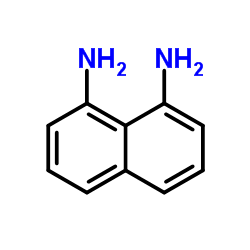
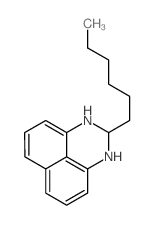
![1,5,8,12-tetrazabicyclo[10.2.2]hexadecane Structure](https://image.chemsrc.com/caspic/291/72952-82-0.png)

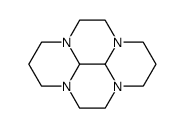
![2-[2-(1,4,5,6-tetrahydropyrimidin-2-yl)ethyl]-1,4,5,6-tetrahydropyrimidine Structure](https://image.chemsrc.com/caspic/017/78706-92-0.png)