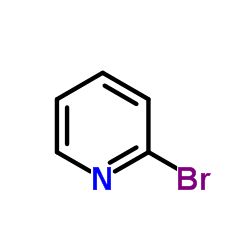
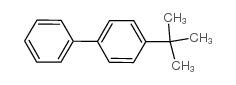
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
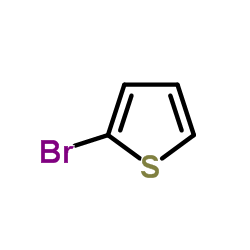
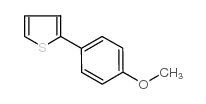

![[1,1'-Biphenyl]-4-amine,N,N-dimethyl Structure](https://image.chemsrc.com/caspic/481/1137-79-7.png)


![METHYL 4'-METHOXY-[1,1'-BIPHENYL]-3-CARBOXYLATE Structure](https://image.chemsrc.com/caspic/276/19617-68-6.png)
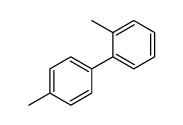
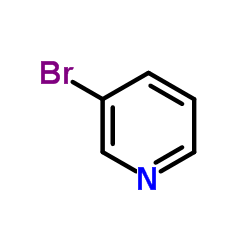
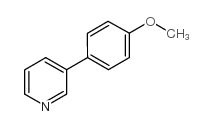
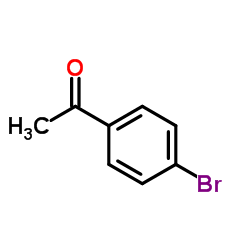
![1-[4-(2-methylphenyl)phenyl]ethanone Structure](https://image.chemsrc.com/caspic/146/56917-39-6.png)
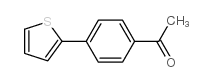
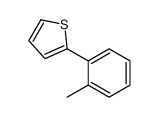
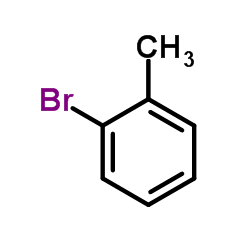

![4'-(TERT-BUTYL)-N,N-DIMETHYL-[1,1'-BIPHENYL]-4-AMINE Structure](https://image.chemsrc.com/caspic/444/98236-17-0.png)
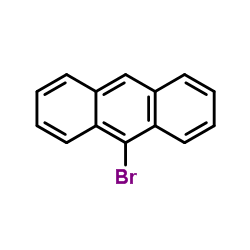
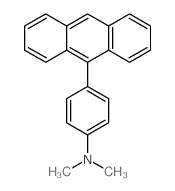
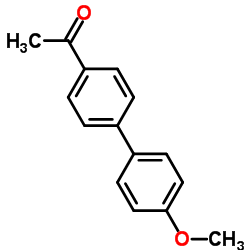

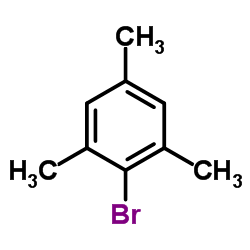
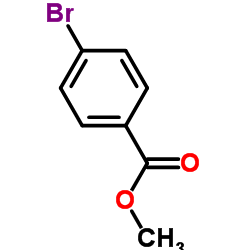
![2'-Methyl-[1,1'-Biphenyl]-4-Carboxylic Acid Methyl Ester Structure](https://image.chemsrc.com/caspic/042/89900-99-2.png)