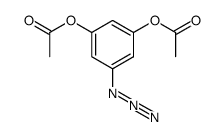
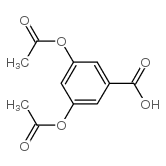
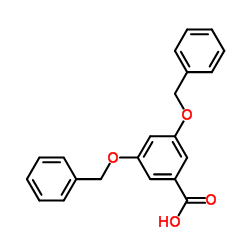
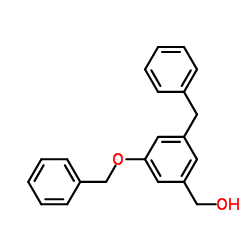
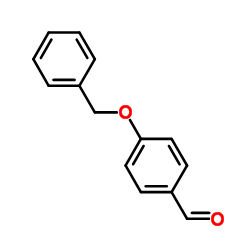
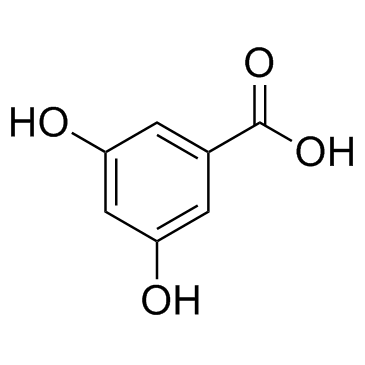
|
~70% |
|
~19% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~55% |
|
~84% |
|
~80% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~85% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

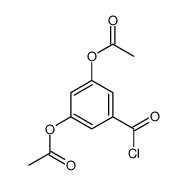
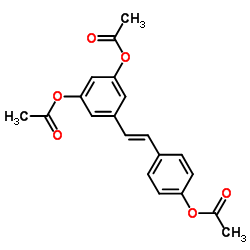


![7-chloro-2-(pyridin-4-yl)benzo[d]thiazole Structure](https://image.chemsrc.com/caspic/399/51642-28-5.png)