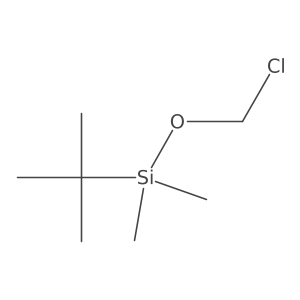
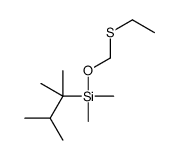
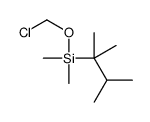
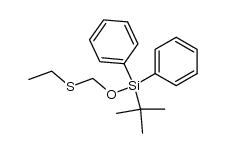
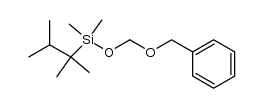
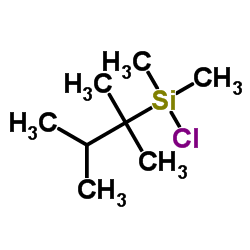
|
~73% |
|
~77% |
|
~66% |
|
~67% |
|
~64% |
|
~72% |
|
~73% |
|
~0% |
|
~0% |
|
~0% |
|
~77% |
|
~80% |
|
~98% |
|
~% |
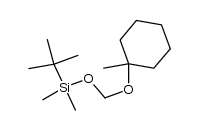
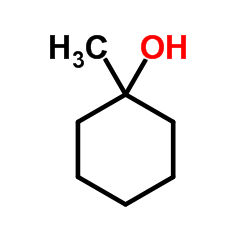

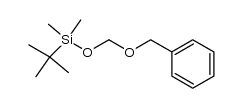

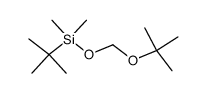
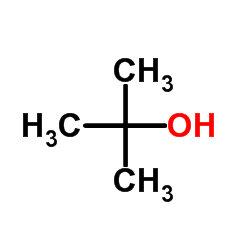
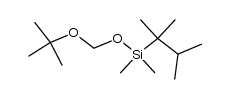
(2-methyl-2-propanyl)silane Structure](https://image.chemsrc.com/caspic/004/119451-79-5.png)