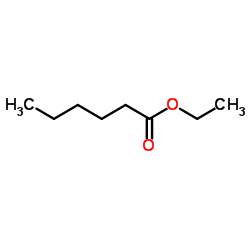
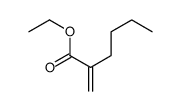
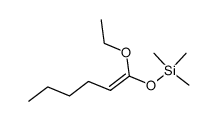
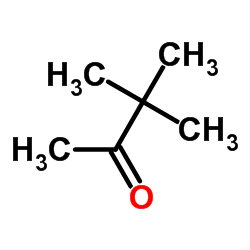
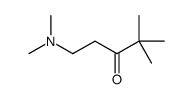
|
~0% |
|
~% |
|
~% |
|
~17% |
|
~% |
|
~0% |
|
~% |
|
~% |
|
~% |
|
~% |

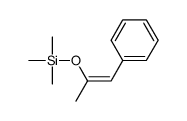
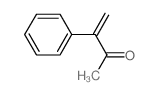
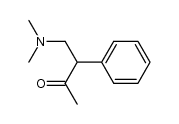
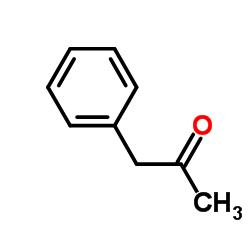
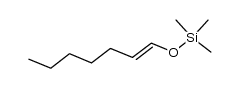
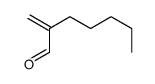
![2-[(dimethylamino)methyl]heptanal Structure](https://image.chemsrc.com/caspic/461/62150-22-5.png)