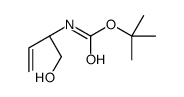
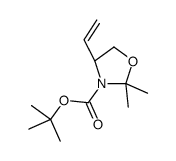
|
~89% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~94% |
![(S)-Methyl 4-[(tert-butoxycarbonyl)amino]-5-hydroxypentanoate Structure](https://image.chemsrc.com/caspic/074/126587-35-7.png)
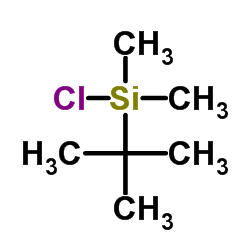
![methyl 5-[tert-butyl(dimethyl)silyl]oxy-4-[(2-methylpropan-2-yl)oxycarbonylamino]pentanoate Structure](https://image.chemsrc.com/caspic/278/96014-55-0.png)
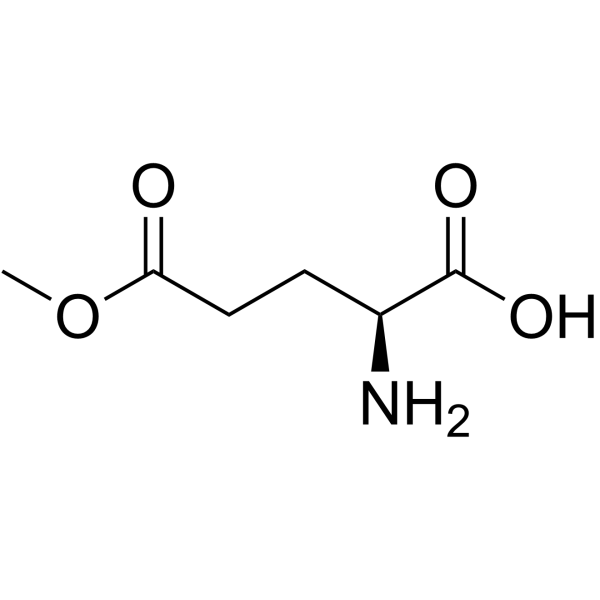


![(5S)-5-[[[(tert-Butyl)dimethylsilyl]oxy]methyl]-2-pyrrolidinone Structure](https://image.chemsrc.com/caspic/052/106191-02-0.png)