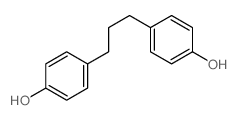
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
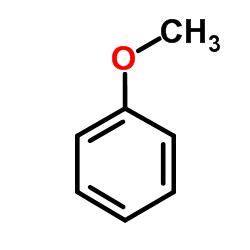
![1-methoxy-4-[6-(4-methoxyphenyl)hexyl]benzene Structure](https://image.chemsrc.com/caspic/229/4280-58-4.png)
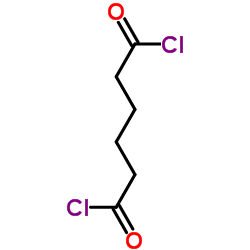
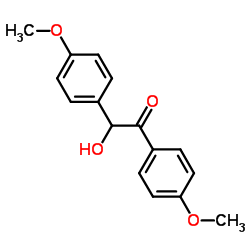
![4-[6-(4-hydroxyphenyl)hexyl]phenol Structure](https://image.chemsrc.com/caspic/028/3682-95-9.png)


![1-methoxy-4-[3-(4-methoxyphenyl)propyl]benzene Structure](https://image.chemsrc.com/caspic/017/4741-73-5.png)