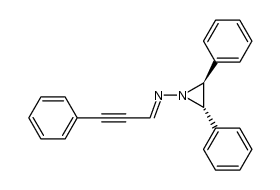
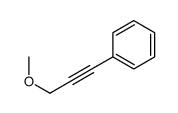
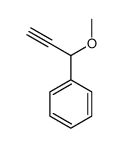
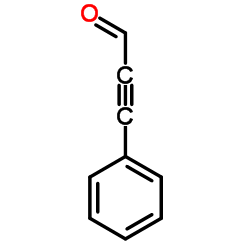
|
~0% |
|
~% |
|
~31% |
|
~% |