|
~% |
|
~% |
|
~% |
|
~% |
|
~90% |
|
~% |
|
~% |
|
~% |
|
~52% |
|
~% |

![2-phenylpyrazolo[5,1-a]isoquinoline Structure](https://image.chemsrc.com/caspic/264/61001-36-3.png)
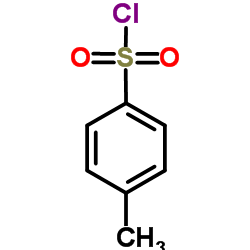
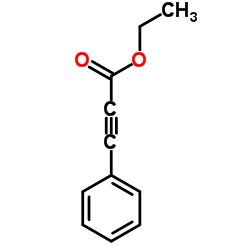
![2-phenyl-pyrazolo[5,1-a]isoquinoline-1-carboxylic acid ethyl ester Structure](https://image.chemsrc.com/caspic/321/63237-71-8.png)
![ethyl 2-phenylpyrazolo[1,5-a]quinoline-3-carboxylate Structure](https://image.chemsrc.com/caspic/152/63237-80-9.png)
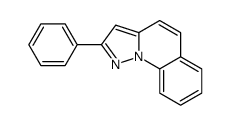

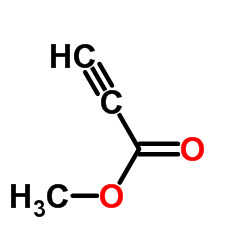
![methyl pyrazolo[1,5-a]quinoline-3-carboxylate Structure](https://image.chemsrc.com/caspic/420/55734-86-6.png)