|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
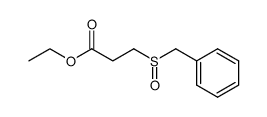
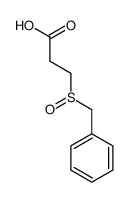

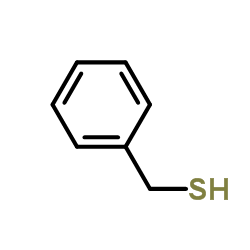
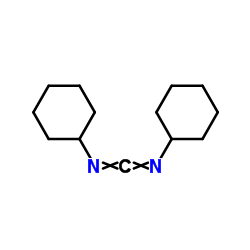
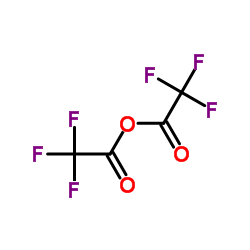
![Acetamide,N-cyclohexyl-N-[(cyclohexylamino)carbonyl]-2,2,2-trifluoro Structure](https://image.chemsrc.com/caspic/305/4706-96-1.png)

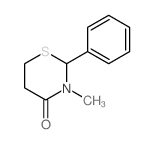
![N-methyl 3-[(phenylmethyl)thio]propanamide Structure](https://image.chemsrc.com/caspic/458/56788-03-5.png)